Several technical challenges must be overcome to make it possible for cellular agriculture — the process of growing meat in bioreactors — to feed millions of people. Muscle cells from chicken, fish, cows, and other food sources must be grown to produce millions of metric tons annually.
Toward this goal, researchers at the Tufts University Center for Cellular Agriculture developed immortalized bovine muscle stem cells (iBSCs) that can proliferate and divide hundreds of times and possibly indefinitely. This advance, described in the journal ACS Synthetic Biology, means that researchers and companies around the globe can have access to and develop new products without repeatedly sourcing cells from farm animal biopsies.
The production of cell-cultured meat will require muscle and fat cells with a very high capacity to grow and divide. While cell-grown meat has garnered media attention with examples such as the FDA preliminary approval of cultured chicken, and even a hamburger has grown with mastodon DNA, the products are still expensive and difficult to scale up.
Normal muscle stem cells drawn from live animals to start a culture typically divide about 50 times before they start to get “old” and are no longer viable.
While it is theoretically possible for these stem cells to produce a substantial amount of meat, the immortalized cells developed by the TUCCA team offer several advantages. One is the possibility of producing significantly more mass for meat production.
Another advantage is that by making the immortalized cells widely available, they will lower the barrier of entry for other researchers to explore cellular agriculture — finding ways to reduce costs and overcome challenges to scaled-up production.
“Typically, researchers have had to do their isolations of stem cells from animals, which is expensive and laborious, or use model cell lines from less relevant species, like mouse muscle cells,” said Andrew Stout, a graduate student at TUCCA and lead researcher on the project, “Using these new persistent bovine cell lines, their studies can be more relevant, literally getting right to the meat of the matter.”
Two steps were key to transforming regular bovine muscle stem cells into immortalized bovine muscle stem cells. As they divide and age, most cells lose DNA at the ends of their chromosomes, called telomeres, like worn ropes that get frayed with use. This can lead to errors when the DNA is being copied or repaired. It can also cause genes to be lost, and cells eventually die.
The researchers engineered the bovine stem cells to constantly rebuild their telomeres, keeping their chromosomes “youthful” and ready for another round of replication and cell division.
The second step to immortalizing the cells was to make them continuously produce a protein that stimulates a critical stage of cell division. This effectively turbocharges the process and helps the cells to grow faster.
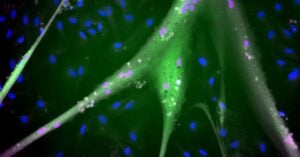
Muscle stem cells are not the final product that one wants to eat. They must divide and grow and differentiate into mature muscle cells like, or at least very similar to, the muscle cells we eat in a steak or fillet.
Stout and his research team found that the new stem cells differentiated into mature muscle cells, although not identical to animal or muscle cells from conventional bovine stem cells.
“It’s possible that they are matured enough to replicate the flavor and texture of natural meat,” said Stout, “That’s something we will have to explore further. They are doubling at a very rapid rate, so they might just need a little more time to reach full maturity.”
“While some may question whether it is safe to ingest immortalized cells, in fact, by the time the cells have been harvested, stored, cooked, and digested, there is no viable path to continued growth,” said David Kaplan, Stern Family professor of biomedical engineering at Tufts and director of TUCCA. “Like natural meat, we eat today, the cells simply become inert material that we hope will taste delicious and provide a wide range of nutritious benefits.”


